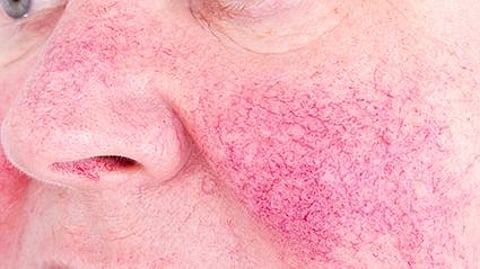
WEDNESDAY, April 17, 2024 (HealthDay News) -- The anticalcitonin gene-related peptide-receptor monoclonal antibody erenumab is effective and safe for treatment of rosacea-associated erythema and flushing, according to a study published online April 17 in JAMA Dermatology.
Nita K.F. Wienholtz, M.D., Ph.D., from Copenhagen University Hospital-Rigshospitalet in Denmark, and colleagues examined the effectiveness, tolerability, and safety of erenumab for treatment of rosacea-associated erythema and flushing in a single-center, nonrandomized trial involving adults with rosacea with at least 15 days of moderate-to-severe erythema and/or moderate-to-extreme flushing. Participants received 140 mg erenumab subcutaneously every four weeks for a period of 12 weeks; safety was assessed at week 20.
Thirty participants were included; 27 completed the study. The researchers observed a reduction of −6.9 days in the mean number of days with moderate-to-extreme flushing, from 23.6 days at baseline. For moderate-to-severe erythema, the mean number of days was reduced by −8.1 from 15.2 at baseline. Adverse events included transient mild-to-moderate constipation, transient worsening of flushing, bloating, and upper respiratory tract infection (33, 13, 10, and 10 percent, respectively). One participant discontinued the study due to a serious adverse event, deemed unrelated to the study, and two withdrew consent due to time constraints.
"The results also suggest that erenumab is generally well tolerated, with mild and/or transient adverse effects," the authors write. "Larger randomized, controlled, double-blind studies are necessary to confirm these findings and to investigate the long-term effects of erenumab treatment."
Several authors disclosed ties to pharmaceutical companies, including Novartis Pharma, which funded the study.
Abstract/Full Text (subscription or payment may be required)
Editor's Note (subscription or payment may be required)